Canvas is matched to your browser window. 15 rows The two-dimensional arrangement of the spheres can be extended to the third dimension to form a.

Face Centered Cubic Fcc A Closest Packing Structure Lattice Structure Crystalline Solid Chemistry
What is Simple Cubic Unit Cell.

. The simple cubic unit cell is the simplest repeating unit in a simple cubic structure. If the atomic mass is known the mass of the unit cell is equal to atomic mass Avogadro number atomic mass 6022 1023g. Use your fingers or mouse to control the model hold shift key or use mouse wheel to zoom it.
Simple Cubic unit cell. The simple cubic unit cell is a cube all sides of the same length and all face perpendicular to each other with an atom at each corner of the unit cell. These are corner atoms so each one only contributes one eighth of an atom to the unit cell thus giving.
The edges of the unit cells are of equal length and the cell angles. These are corner atoms so each one only contributes one eighth of an atom to the unit cell thus. The unit cell completely describes the.
The unit cell commonly selected for a simple cubic metal is a cube because this is the simplest repeating part of the lattice. This chemistry video tutorial provides a basic introduction into unit cell and crystal lattice structures. The total of atoms located in the End-Centred Cubic Unit Cell 1 1 2 atoms.
The simple cubic unit cell is a cube all sides of the same length and all face perpendicular to each other with an atom at each corner of the unit cell. It highlights the key differences between the sim. Each atom is making a contribution of 12th cells portionpart.
At the cells centre two atoms are present. A simple cubic unit cell contains one atom. The Density of Simple Cubic Unit Cell formula is defined as the ratio of mass of all atoms to the volume of unit cell and is represented as ρ M V unit cell Avaga-no or Density Mass of.
19 rows Simple Cubic Unit Cell is the least complex cubic unit cell and contains only 1 atom. Simple Cubic Unit Cell. Each corner of the unit cell is defined by a lattice point at which an.
The simple cubic unit cell is delineated by eight atoms which mark the actual cube. The simple cubic unit cell is delineated by eight lattice points which mark the actual cube.

Face Centered Cubic Fcc Unit Cell Earrings For Materials Scientists Unit Cell Materials Scientist Bravais Lattice

Unit Cell Chemistry Atomic Radius Density Edge Length Calculations Unit Cell The Unit Atom

Unit Cell Chemistry Atomic Radius Density Edge Length Calculations Unit Cell Chemistry The Unit
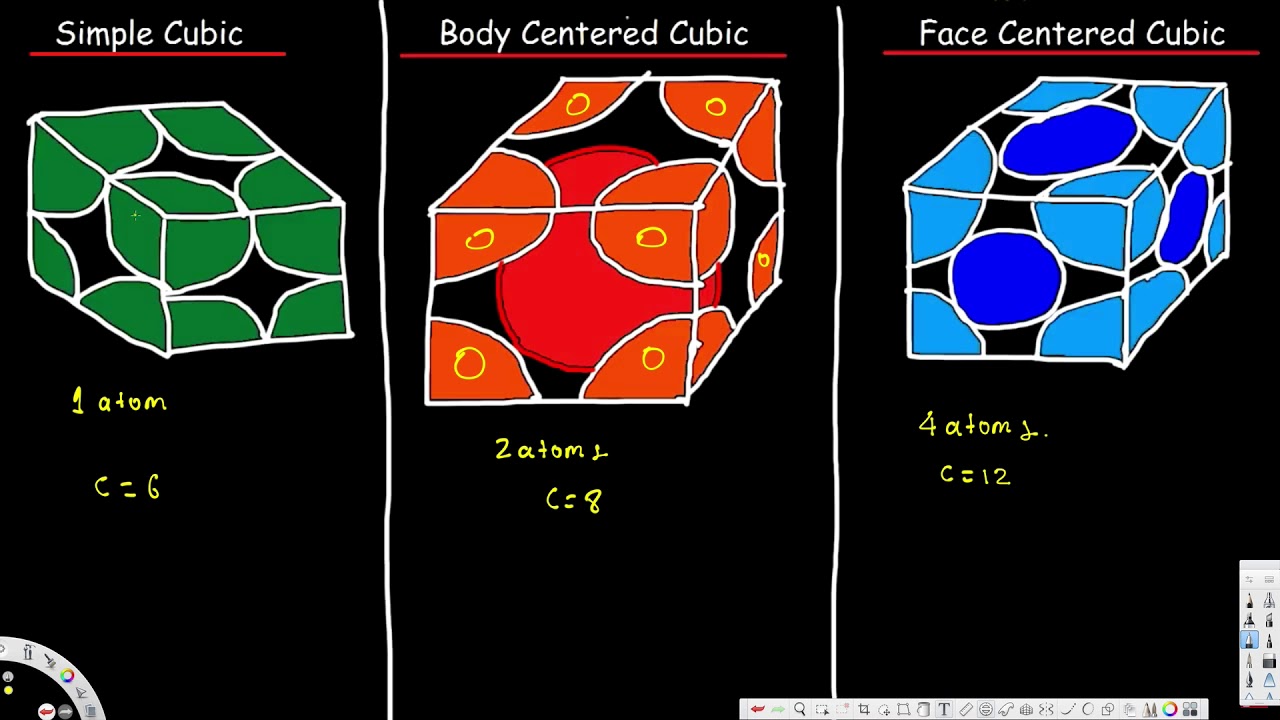
Unit Cell Simple Cubic Body Centered Cubic Face Centered Cubic Cryst Unit Cell Crystal Lattice Structure Nomenclature Chemistry

Basic Crystal Concepts Bravais Lattice Unit Cell Crystal Lattice

Unit Cell Body Centered Cubic Crystal Lattice Structures Physical E Crystal Lattice Structure Unit Cell Nomenclature Chemistry

Pin By Flathorn On Sacred Geometry Bravais Lattice Chemistry Chemistry Education

Body Centered Cubic Model In Silver Interestingly The Bcc Unit Cell Was The Inspiration For The Atomium In Belgium But The Unit Cell Science Decor Crystals

Chemistry Liquids And Solids 32 Of 59 Crystal Structure Seven Types Of Unit Cells Crystal Structure Chemistry Unit Cell

11 7 Structure Of Solids Chemistry Libretexts Unit Cell Material Science Physical Chemistry

Cubic Lattice From Wolfram Mathworld Cell Forms Unit Cell Lattice

Unit Cell Chemistry Atomic Radius Density Edge Length Calculations Unit Cell Nomenclature Chemistry Chemistry

Unit Cell Face Centered Cubic Crystal Lattice Structures Crystal Lattice Structure Unit Cell Nomenclature Chemistry

Unit Cell Simple Cubic Structure Physical Electronics In 2021 Unit Cell Physics The Unit

Types Of Unit Cell Unit Cell The Unit Cell

Primitive And Non Primitive Unit Cell In 2022 Unit Cell The Unit Primitive

Ncert Solutions For Class 12 Chemistry Chapter 1 The Solid State Cbse Tuts Class12chemistrychapter1ncertsolutions Chemistry Ap Chemistry Solutions


